Reactivity of triplet carbenes explained with a new nonadiabatic tunnelling mechanism
Instanton theory reveals a crossover between a sequential and a concerted tunelling mechanism.
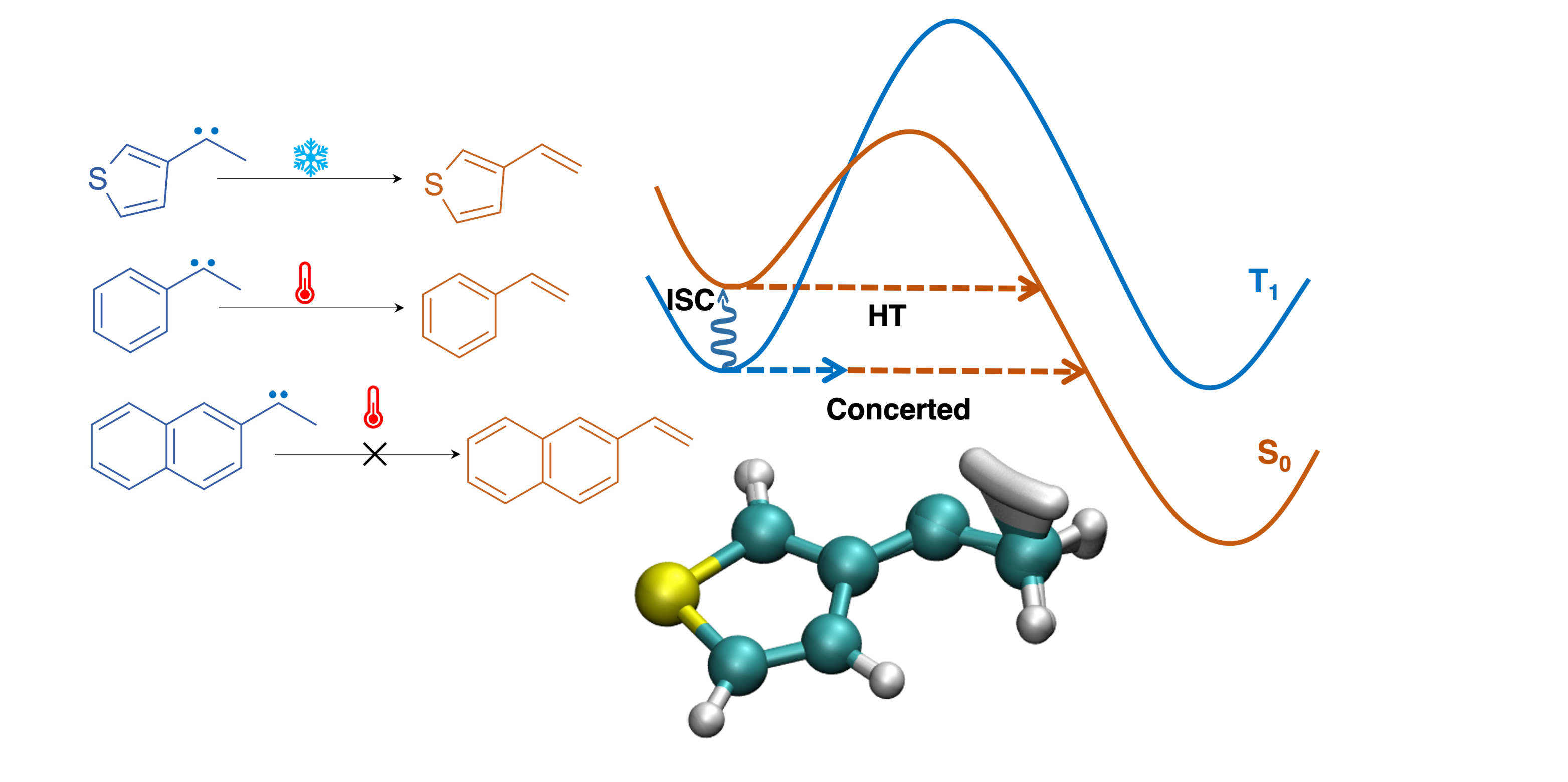
Instanton theory was used to study the intramolecular hydrogen transfer (HT) reaction in triplet carbenes to give singlet products. Since the reaction involved two steps, HT and intersystem crossing, we looked at two possible mechanisms: a sequential and an as yet unknown concerted mechanism. Interestingly, the sequential mechanism governs the reaction at high temperatures while the concerted mechanism dominates at low temperatures, thus explaining the experimentally observed temperature dependence in the reactivity.
Three very similar triplet carbenes showed temperature-dependent reactivity when probed using matrix-isolation experiments. The reaction under study was intramolecular hydrogen transfer (HT) with the product being formed in the singlet state. Therefore, two process HT and intersystem crossing (ISC) are involved. One of the carbenes, C1, reacted at 10 K, while another, C2, required heating to 65 K. The third carbene, C3, showed no reactivity despite heating.
We considered a sequential (where ISC is followed by HT) and a concerted (where both happen simultaneously) pathway as possible reaction mechanisms. The aim was to use instanton theory to evaluate these possibilities by calculating their rates and comparing with experimental values. Adiabatic and nonadiabatic instanton theories were used to calculate the rate of the sequential pathway. However, the concerted mechanism is a new type of reaction mechanism which has previously not been studied, and required the development of a new type of instanton to describe it.
We found that both channels exist in all three carbenes, but dominate the reaction mechanism at different temperature regimes. The concerted mechanism dominates the reaction at low temperatures, while the sequential mechanism comes into play at higher temperatures. This gives rise to a crossover temperature in all three carbenes where the mechanism can switch, and also explains why C2 required heating to show any observable reactivity. This study puts forth the appealing possibility of using temperature to modify the reactivity of triplet carbenes.
“Angew. Chem. Int. Ed. 2025, e2503066. https://doi.org/10.1002/anie.202503066”M.A. Manae, J.O. Richardson